Our technology
Contguard specializes in advanced supply chain risk management and in-transit visibility through its innovative software, CGI (Contguard Insights), and cutting-edge IoT devices. Designed to support global enterprises, our holistic solution offers real-time data on your goods including precise location, risk assessment, and quality monitoring. Receive immediate proactive alerts to ensure effective safeguarding of your valuable shipments.
read more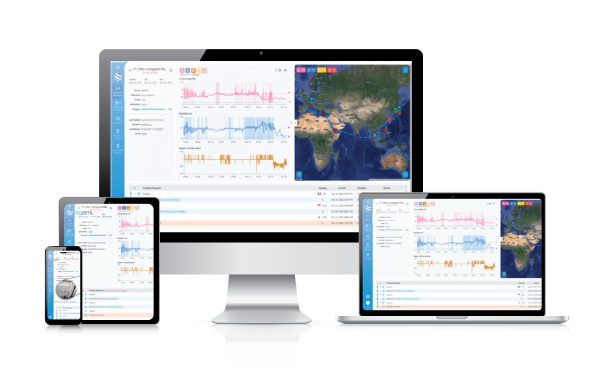
Our SOLUTIONS
Transform supply chain management with Contguard’s advanced solutions for risk mitigation and in-transit transparency. Our innovative approach not only ensures the security of your goods during transit but also enables supply chain optimization. With continuous full-time support, our solutions provide peace of mind regarding your cargo’s state throughout its journey.
Risk Management for
Supply Chain
Elevate cargo integrity with real-time security monitoring, theft prevention, and quality assurance.
read more
24/7/365
Escalation Center
Contguard’s dedicated 24/7 control center team offers full-time support. Their extensive training and constant availability enables swift real-time responses when necessary.
read moreSupply Chain
Optimization
Optimize supply chain efficiency through detailed route analysis, accurate AI-based ETAs, and cost-effective demurrage management.
read moreFull Reverse Logistics
of IoT Devices
Contguard's full reverse logistics for IoT devices guarantees smooth deployment and operational efficiency for businesses around the world.
read moreCarbon Emissions
Analysis
Contguard’s Scope 3 carbon emissions analysis contributes to sustainability efforts while enhancing supply chain efficiency.
read more


Sustainability
Contguard is unwavering in its commitment to environmental sustainability, recognizing the pivotal role of energy conservation in achieving this mission. Our proactive efforts are aimed at contributing to a greener world through conscientious practices and innovative solutions.
Recent updates
Who we are
Contguard is a global AI-based IoT technology company that provides advanced solutions for securing and monitoring goods in transit. With over a decade of collaboration with global organizations, we are dedicated to empowering businesses worldwide. Our mission is clear: to deliver an end-to-end service that ensures complete visibility, security, and sustainability of your goods during transit. Our holistic approach is designed to safeguard the integrity of your valuable assets while optimizing the efficiency of your supply chain operations.